ISO 6976-16
Compression Factor
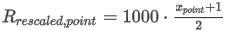
where the summation is taken over all N components of the mixture and:
Z(t2,p2) is the compression factor at metering temperature t2 and metering pressure p2
p0 is the atmospheric pressure (101.325 kPa)
p2 is the metering pressure
xj is the mole fraction of component j
s(t2,p0) is the summation factor for component j at metering temperature t2 and atmospheric pressure p0
Mean Molecular Weight

where the summation is taken over all N components of the mixture and:
M is the mean molecular weight
xj is the mole fraction of component j
Mj is the molar mass of component j
Gross Calorific Value
- Molar basis (real gas property is calculated as ideal)

where the summation is taken over all N components of the mixture and:
(Hc)0G(t1) is the ideal molar gross calorific value of the mixture at combustion temperature t1
[(Hc)0G]j(t1) is the ideal molar gross calorific value of the component j at combustion temperature t1
xj is the mole fraction of component j
- Mass basis (real gas property is calculated as ideal)

where:
(Hm)0G(t1) is the ideal mass gross calorific value of the mixture at combustion temperature t1
(Hc)0G(t1) is the ideal molar gross calorific value of the mixture at combustion temperature t1
M is the mean molecular weight
- Volumetric basis - ideal gas

where:
(Hv)0G(t1;t2,p2) is the ideal volumetric gross calorific value of the mixture at combustion temperature t1
(Hc)0G(t1) is the ideal molar gross calorific value of the mixture at combustion temperature t1
p2 is the metering pressure
R is the molar gas constant
T2 is the absolute metering temperature in kelvins
- Volumetric basis - real gas

where:
(Hv)G(t1;t2,p2) is the real volumetric gross calorific value of the mixture at combustion temperature t1
Net Calorific Value
- Molar basis (real gas property is calculated as ideal)

where the summation is taken over all N components of the mixture and:
(Hc)0N(t1) is the ideal molar net calorific value of the mixture at combustion temperature t1
(Hc)0G(t1) is the ideal molar gross calorific value of the mixture at combustion temperature t1
xj is the mole fraction of component j
bj is the hydrogen index of component j
L0(t1) is the standard enthalpy of vaporization of water at combustion temperature t1
- Mass basis (real gas property is calculated as ideal)
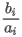
where:
(Hm)0N(t1) is the ideal mass net calorific value of the mixture at combustion temperature t1
(Hc)0N(t1) is the ideal molar net calorific value of the mixture at combustion temperature t1
M is the mean molecular weight
- Volumetric basis - ideal gas
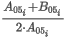
where:
(Hv)0N(t1;t2,p2) is the ideal volumetric net calorific value of the mixture at combustion temperature t1
(Hc)0N(t1) is the ideal molar net calorific value of the mixture at combustion temperature t1
p2 is the metering pressure
R is the molar gas constant
T2 is the absolute metering temperature in kelvins
- Volumetric basis - real gas

where:
(Hv)N(t1;t2,p2) is the real volumetric net calorific value of the mixture at combustion temperature t1
Relative Density
- Ideal gas

where:
G0 is the relative density of the ideal gas
M is the mean molecular weight
Mair is the molar mass of dry air of standard composition (28.96546 kg·kmol-1)
- Real gas

where:
G(t2,p2) is the relative density of the real gas at t2 and p2
Z(t2,p2) is the compression factor of the gas mixture at t2 and p2
Zair(t2,p2) is the compression factor of dry air of standard composition at t2 and p2 calculated as:
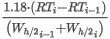
where Zair(t2,p0) is the compression factor of the dry air of standard composition at t2 and p0
Density
- Ideal gas
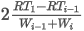
where:
D0(t2,p2) is the density of the ideal gas at t2 and p2
M is the mean molecular weight
R is the molar gas constant
T2 is the absolute metering temperature in kelvins
- Real gas
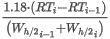
where:
D(t2,p2) is the density of the real gas at t2 and p2
Z(t2,p2) is the compressibility factor of the gas mixture at t2 and p2
Wobbe Index (Gross and Net)
- Ideal gas
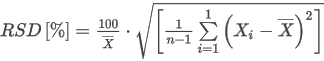
where:
W0G/N(t1;t2,p2) is the gross or net Wobbe index of the ideal gas at combustion temperature t1
(Hv)0G/N(t1;t2,p2) is the ideal volumetric gross or net calorific value of the mixture at combustion temperature t1
G0 is the relative density of the ideal gas
- Real gas

where:
WG/N(t1;t2,p2) is the gross or net Wobbe index of the real gas at combustion temperature t1
(Hv)G/N(t1;t2,p2) is the real volumetric gross or net calorific value of the mixture at combustion temperature t1
G is the relative density of the real gas
ISO 6976-95
Compression Factor

where the summation is taken over all N components of the mixture and:
Zmix is the compression factor
xj is the mole fraction of component j
√bj is the summation factor for component j
Mean Molecular Weight
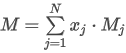
where the summation is taken over all N components of the mixture and:
M is the mean molecular weight
xj is the mole fraction of component j
Mj is the molar mass of component j
Superior and Inferior Calorific Value
- Molar basis (real gas property is calculated as ideal)
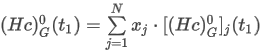
where the summation is taken over all N components of the mixture and:
H0(t1) is the ideal molar calorific value of the mixture (superior or inferior)
Hj0(t1) is the ideal molar calorific value of the component j (superior or inferior)
xj is the mole fraction of component j
- Mass basis (real gas property is calculated as ideal)
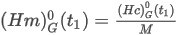
where:
Ĥ0(t1) is the ideal calorific value on a mass basis of the mixture (superior or inferior)
H0(t1) is the ideal molar calorific value of the mixture (superior or inferior)
M is the mean molecular weight
- Volumetric basis - ideal gas

where:
H̃0[t1,V(t2,p2)] is the ideal calorific value on a volumetric basis, for a combustion temperature t1, of the mixture (superior or inferior), metered at a temperature t2 and pressure p2
H0(t1) is the ideal molar calorific value of the mixture (superior or inferior)
R is the molar gas constant
T2 is the absolute temperature in kelvins
- Volumetric basis - real gas
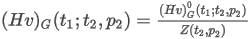
where:
Zmix(t2,p2) is the compression factor at the metering reference conditions
Relative Density
- Ideal gas
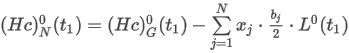
where the summation is taken over all N components of the mixture and:
d0 is the relative density of the ideal gas
Mj is the molar mass of component j
Mj is the molar mass of dry air of standard composition
- Real gas
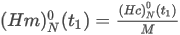
where the summation is taken over all N components of the mixture and:
d is the relative density of the real gas
Zmix is the compression factor of the gas mixture
Zair is the compression factor of dry air of standard composition
Density
- Ideal gas

where the summation is taken over all N components of the mixture and:
ρ0(t,p) is the density of the ideal gas
R is the molar gas constant
T is the absolute temperature in kelvins
xj is the mole fraction of component j
Mj is the molar mass of component j
- Real gas
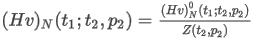
where:
ρ(t,p) is the density of the real gas
Zmix is the compressibility factor of the gas mixture
Wobbe Index
- Ideal gas
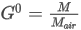
where:
W0[t1,V(t2,p2)] is the Wobbe index of the ideal gas
d0 is the relative density of the ideal gas
- Real gas

where:
W[t1,V(t2,p2)] is the Wobbe index of the real gas
d(t2,p2) is the relative density of the real gas
ASTM D 3588-98 and GPA 2172-19
Molar Mass
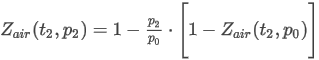
where the summation is taken over all N components of the mixture and:
M is the molar mass of the mixture
xj is the mole fraction of component j
Mj is the molar mass of component j
Molar Mass Ratio
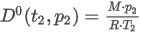
where the summation is taken over all N components of the mixture and:
Gid is the molar mass ratio of the mixture
xj is the mole fraction of component j
Gjid is the molar mass ratio of the compound j
M is the molar mass of the mixture
Ma is the molar mass of air
Compressibility Factor
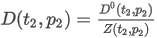
where the summation is taken over all N components of the mixture and:
Z(T,P) is the compression factor
P is the pressure
xj is the mole fraction of component j
√βj is the summation factor for component j
Relative Density
- Ideal gas

where:
did is the relative density of the ideal gas
M is the molar mass of the mixture
Ma is the molar mass of air
- Real gas
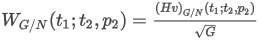
where:
d is the relative density of the real gas
Z is the compression factor of the gas mixture
Za is the compression factor of dry air
M is the molar mass of the mixture
Ma is the molar mass of air
Heating Value
According to ASTM D 3588-98, the real heating value is not given by division of the ideal heating value by the compressibility factor (Z). Real gas heating values differ from the ideal gas values by less than the order of the accuracy of the heating values. Thus, the real heating value is calculated as ideal. Furthermore, according to GPA 2172-19, dividing the ideal volumetric heating value by the compressibility factor provides the energy transferred in an ideal gas reaction per volume of real gas fuel (in Clarity, printed as Ideal Heating Value per Real Gas Volume).
- Molar basis
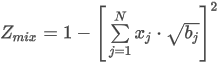
where the summation is taken over all N components of the mixture and:
Hnid is the heating value of the gas mixture on molar basis (gross or net)
xj is the mole fraction of component j
Hn,jid is the molar heating value of component j (gross or net)
- Mass basis
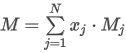
where the summation is taken over all N components of the mixture and:
Hmid is the heating value of the gas mixture on mass basis (gross or net)
xj is the mole fraction of component j
Mj is the molar mass of component j
Hm,jid is the heating value per unit mass of component j (gross or net)
- Volumetric basis
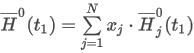
where the summation is taken over all N components of the mixture and:
HVid is the heating value of the gas mixture on volumetric basis (gross or net)
xj is the mole fraction of component j
HV,jid is the heating value per unit volume of component j (gross or net)
ASTM D 2421-02 and D 2598-02
Vapor Pressure
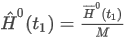
where the summation is taken over all N components of the mixture and:
pV is the liquified petroleum (LP) gas vapor pressure of the sample at 37.8 °C
xV,j is the liquid volume percent of component j in the mixture
vpj' is the vapor pressure factor of component j at 37.8 °C
Relative density

where the summation is taken over all N components of the mixture and:
d is the relative density of the mixture
xV,j is the liquid volume percent of component j in the mixture
sgj' is the relative density of component j at 15.6 °C
Motor Octane Number
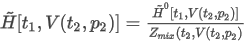
where the summation is taken over all N components of the mixture and:
MON is the calculated motor octane number of the mixture
xV,j is the liquid volume percent of component j in the mixture
monj is the motor octane number of component j
ISO 8973-97 and EN 589+A1
Density

where the summation is taken over all N components of the mixture and:
ρ is the density of the mixture at 15 °C
ρj is the density factor of component j in the mixture at 15 °C
Wj' is the mass fraction of component j in the mixture, calculated as:

where the summation is taken over all N components of the mixture and:
xj is the mole fraction of component j
Mj is the relative molecular mass of component j
Absolute Vapor Pressure
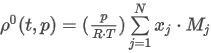
where the summation is taken over all N components of the mixture and:
pv is the absolute vapor pressure of the LPG sample
xj is the mole fraction of component j in the mixture
pv,j is the vapor pressure factor of component j
Gauge Vapor Pressure

where:
pve is the gauge vapor pressure
Octane Number
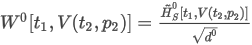
where the summation is taken over all N components of the mixture and:
ON is the calculated octane number of the mixture
xj is the molar fraction of component j in the mixture
ponj is the partial octane number of component j
Additional notes
Methane Number
According to KUBESH, John; KING, Steven R.; LISS, William E. Effect of gas composition on octane number of natural gas fuels. SAE Technical Paper, 1992, equation (4):
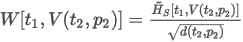
where:
MN is the calculated methane number of the mixture
ON is the octane number of the mixture
1.624 and 119.1 are the coeficients obtained via regression as described in the Article
Calculation on wet basis
For calculations of gas properties on wet basis according to norms ISO 6976-95, ASTM D 3588-98, and GPA 2172-19, the mole fraction of water in natural gas saturated with water is added to the resulting NGA Amounts. For ASTM D 3588-980 and GPA 2172-19, the base temperature is defined as 60 °F (corresponding to the saturated mole fraction of water equal to 0.01744). In ISO 6976-95, the available metering temperatures are 0, 15, and 20 °C. The mole fractions of water vapor in saturated gas at these temperatures were calculated according to the following equation:
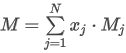
where:
xw(T) is the mole fraction of water vapor in saturated gas at temperature T
patm is the atmospheric pressure (101.325 kPa).
psat(T) is the saturation vapor pressure of water at temperature T, obtained from the NIST database, Saturation water properties, P.J. Linstrom and W.G. Mallard, Eds., NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD, 20899, https://doi.org/10.18434/T4D303, (retrieved February 15, 2024).